Our research
The DUOXLab – From H2O2 generator DUOX to oxidative stress and cancer development.
Our research group is part of the Institute of Interdisciplinary Research, IRIBHM (Institut de Recherche Interdisciplinaire en Biologie Humaine et Moléculaire). The Institute is localized at the Medical School of the University of Brussels (Université Libre de Bruxelles, ULB).
Hydrogen peroxide (H2O2) is a highly reactive chemical molecule originally considered as an antibacterial agent. In excess, H2O2 induces an oxidative stress responsible for different pathologies including cardiovascular diseases, neurodegeneration, tumorigenesis or ageing. It is now widely accepted that many cell types, other than the phagocytes, produce reactive oxygen species (ROS).
The thyroid and hydrogen peroxide.
 The main function of the thyroid is the uptake and concentration of iodide via the Na+/I- symporteur (NIS) for thyroid hormone biosynthesis. Thyroid hormones, tri- and tetra-iodothyronine (T3 and T4), results from thyroperoxidase (TPO)-catalyzed iodination of thyroglobulin (TG) tyrosine residues and coupling of iodotyrosines. The thyroid hormones act on multiple organs in the body and influence critical physiological processes. They increase the metabolic rate and affect protein synthesis modulating the human body thermoregulation. Foremost, they are absolutely required for normal growth and neuronal maturation, preventing cretinism.
The main function of the thyroid is the uptake and concentration of iodide via the Na+/I- symporteur (NIS) for thyroid hormone biosynthesis. Thyroid hormones, tri- and tetra-iodothyronine (T3 and T4), results from thyroperoxidase (TPO)-catalyzed iodination of thyroglobulin (TG) tyrosine residues and coupling of iodotyrosines. The thyroid hormones act on multiple organs in the body and influence critical physiological processes. They increase the metabolic rate and affect protein synthesis modulating the human body thermoregulation. Foremost, they are absolutely required for normal growth and neuronal maturation, preventing cretinism.
![]()
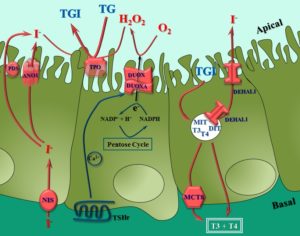
The thyroid gland generates massive amounts of H2O2 required for the biosynthesis of thyroid hormones. In 2000, we have discovered and characterized the molecular nature of the proteins responsible for this thyroid H2O2-generating system: the DUOX1 and DUOX2 enzymes. These proteins belong to a novel protein family, the NADPH-oxidases, presenting a catalytic domain involved in ROS generation.
![]()
Our multidisciplinary projects aim to better characterize the function of these new oxidases and to study their physiological roles using human biological samples and transgenic animal models. Our research group tries to elucidate the mechanisms governing the processing and maturation of DUOX enzymes allowing their correct addressing at the cell surface. Moreover, using molecular approaches, the interactions between DUOX and their maturation factors DUOXA that influence the type of ROS generated (H2O2 or O2.-) are investigated.
![]()
In vivo investigation of DUOX/DUOXA physiological roles: Congenital hypothyroidism and transgenic mouse models
Congenital hypothyroidism (CH) is the most common congenital endocrine disorder affecting 1 in every 3000 newborns. In 20% of the patients, permanent CH is due to biochemical defects causing dyshormonogenesis. Transient CH is often associated with a temporally limited exposure to external factors during pregnancy such as a la ck or an excess of iodide intake, transplacental antibodies, or antithyroid drug-based treatments.
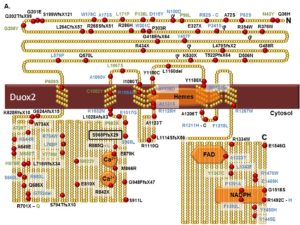
The first genetic basis for transient CH has been proposed with the discovery of affected patients bearing mutations in DUOX2 gene.Several published DUOX2 missense mutations have been characterized in the laboratory. We have demonstrated that they cause DUOX expression and/or maturation defects resulting in the absence or reduced H2O2 generation. Recently, we identified the first inactivating DUOX2 mutation localized in the catalytic core of the oxidase. In collaboration with Pr. G Van Vliet (University of Montreal, Canada) and Pr. DP Carvalho (University of Rio de Janeiro, Brazil), we continue to screen for natural DUOX/DUOXA mutations in CH patients to help for genetic counseling.
![]()
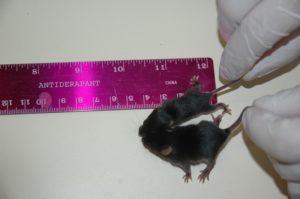
In collaboration with several academic groups around the world, we have also access to transgenic mice very useful to analyze the physiological roles of these novel H2O2 generators in the thyroid metabolism, but also in the other DUOX expressing tissues.
![]()
Excess of H2O2: Risk factor in cancer development ?
Up to 50% of the population above 60 years old present thyroid nodules and 5% of these nodules will degenerate into cancers. Irradiation is the only environmental risk factor clearly implicated in thyroid cancer pathogenesis. However, irradiation is certainly not responsible for the majority of thyroid tumors. We hypothesized that the elevated frequency of thyroid tumors could be partially explained by the mutagenic environment present in the thyroid, resulting from the hormonogenic hydrogen peroxide.
 We have demonstrated that non-lethal concentrations of H2O2 provoke multiple mutagenic DNA double strand breaks in primo-cultured human thyrocytes. We have also shown that DNA breaks induced by H2O2 were more slowly repaired than those induced by irradiation supporting the hypothesis that generation of H2O2 in thyroid could also play a role in mutagenesis particularly in case of antioxidant defense deficiency .
We have demonstrated that non-lethal concentrations of H2O2 provoke multiple mutagenic DNA double strand breaks in primo-cultured human thyrocytes. We have also shown that DNA breaks induced by H2O2 were more slowly repaired than those induced by irradiation supporting the hypothesis that generation of H2O2 in thyroid could also play a role in mutagenesis particularly in case of antioxidant defense deficiency .
![]()
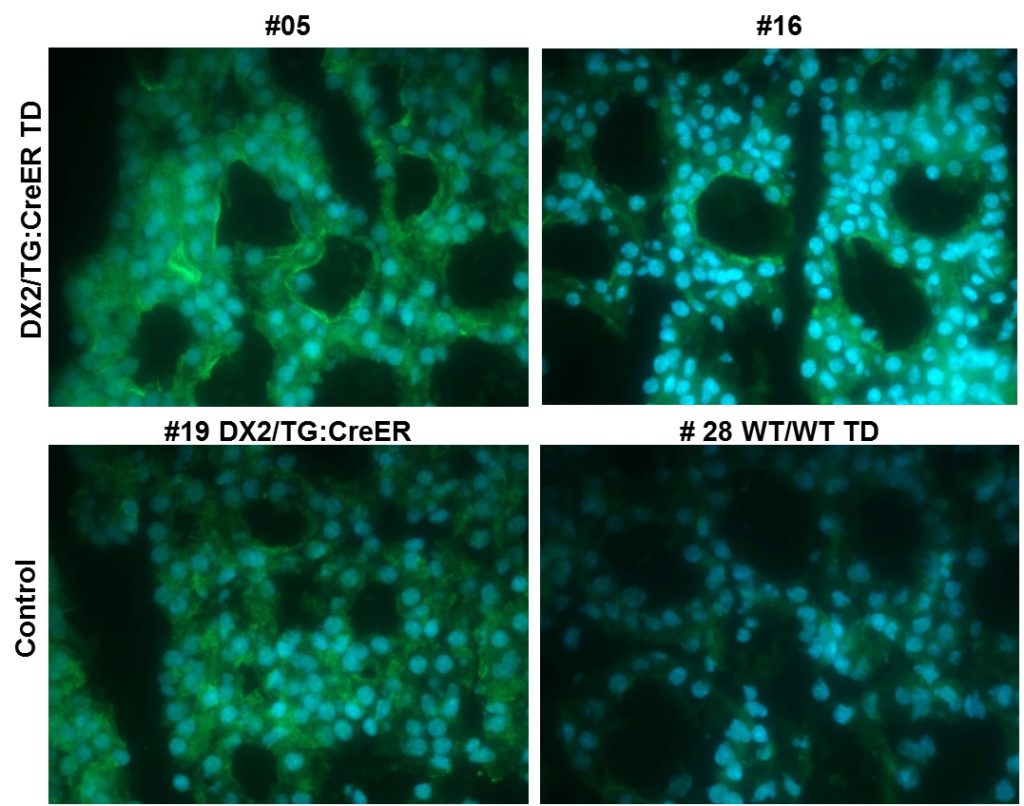
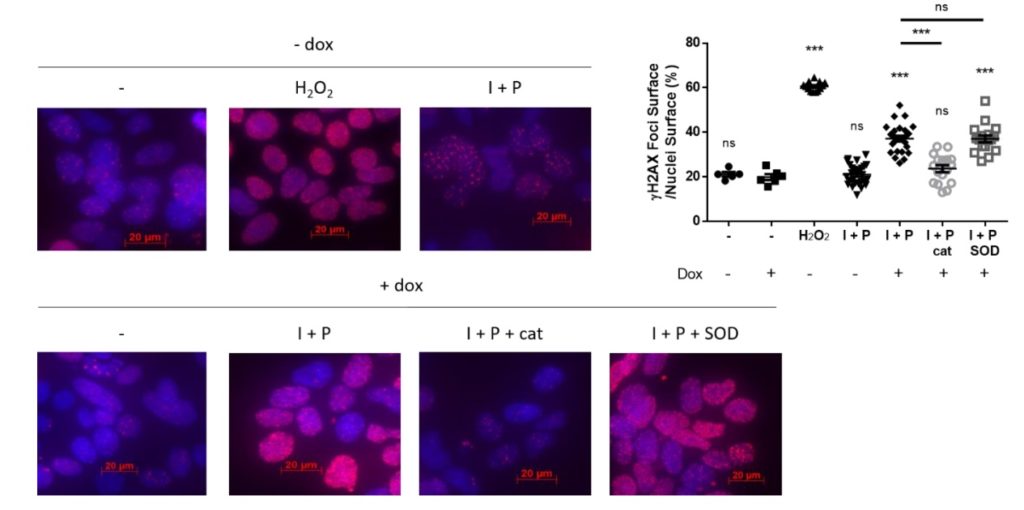 Based on HEK293 Tet-On3G cell lines, we have demonstrated an increase of DNA damage in the nuclei of DUOX2/DUOXA2 expressing cells after doxycycline induction of active DUOX2/DUOXA2 complex (Figure 1). This increase of H2AX phosphorylation was reverted by ROS scavenging using catalase but not superoxide dismutase; and was depended on the level of DUOX2 activation and duration. These data demonstrate forthe first time that H2O2 produced by the DUOX2/DUOXA2 cell surface enzymatic complex could provoke potential mutagenic DNA breaks that may promote in certain pathological conditions the emergence of neoplastic cells. Our hypothesis is that overexposure of the thyroid to a constant insult of hydrogen peroxide will have a profound impact on the function of the thyrocytes and could in some circumstances promote tumor lesions.
Based on HEK293 Tet-On3G cell lines, we have demonstrated an increase of DNA damage in the nuclei of DUOX2/DUOXA2 expressing cells after doxycycline induction of active DUOX2/DUOXA2 complex (Figure 1). This increase of H2AX phosphorylation was reverted by ROS scavenging using catalase but not superoxide dismutase; and was depended on the level of DUOX2 activation and duration. These data demonstrate forthe first time that H2O2 produced by the DUOX2/DUOXA2 cell surface enzymatic complex could provoke potential mutagenic DNA breaks that may promote in certain pathological conditions the emergence of neoplastic cells. Our hypothesis is that overexposure of the thyroid to a constant insult of hydrogen peroxide will have a profound impact on the function of the thyrocytes and could in some circumstances promote tumor lesions.
![]()
To explore this hypothesis in vivo, we have recently generated a new transgenic mouse model overexpressing the enzymatic H2O2 generator DUOX2/DUOXA2 complex (fig. 2). The transgenic animals combine the conditional Cre/LoxP and the inducible Tet-On systems allowing tight modulation of H2O2 exposure in a tissue specific manner. To favor carcinogenesis, transgenic animals will be treated with anti-thyroid drugs or selenium-deficient diets, and by crossbreeding with validated thyroid tumor mouse model.![]()
Characterization of the thyroid-H2O2 generating system in the zebrafish model
Zebrafish has been recognized as an attractive organism to study many biological processes on vertebrate development.
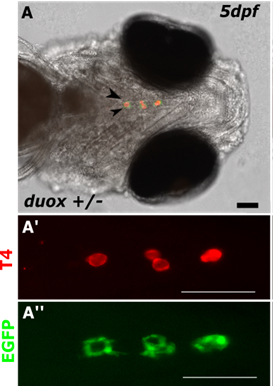
The zebrafish thyroid is not organized in one compact gland encapsulated by connective tissue but consist of follicles of variable shape and diameter that lie individually between the first gill arch and the bulbus arteriosus along the ventral aorta. Compared to the two DUOX and two DUOXA genes present in mammals, only one duox and one single duoxa gene have been identified in the zebrafish genome. Expression of duox mRNA has been demonstrated in various extra-thyroid tissues including the brain, the epidermis, the intestine and the ultimobranchial bodies. In collaboration with the Costagliola Lab (Pr. S. Costagliola), we explored the role of duox and duoxa in thyroid function during zebrafish development using transgenic fish lines invalidated for the related genes.
![]()
Autoimmune Thyroid Disease: Impact of intrathyroidal IL-4 expression
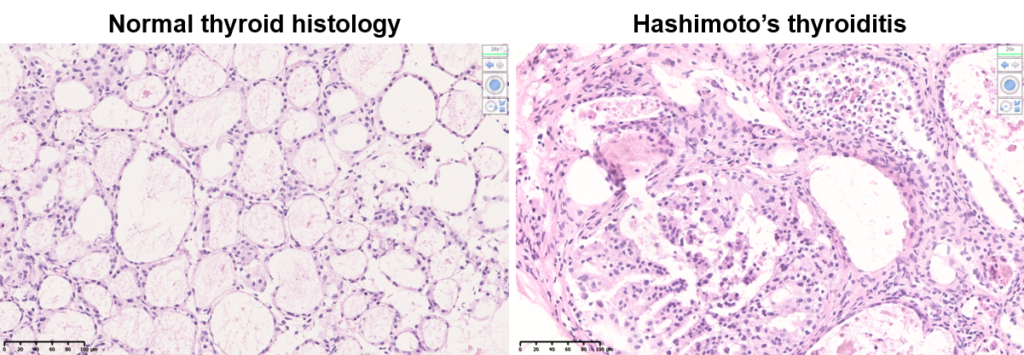
Autoimmune thyroid diseases (AITD) are the most frequent organ-specific autoimmune diseases affecting 3 to 10% of the world population. Hashimoto’s thyroiditis patients develop hypothyroidism as a consequence of a cellular immune response with production of anti-TG autoantibodies, while patients with Graves’ disease (GD) suffer from hyperthyroidism caused by TSHr activating-antibodies. Pro-inflammatory molecules secreted during the inflammation process repress thyroid hormonogenesis mainly by inhibiting the expression of TPO, TG, and NIS. However, the direct effects mediated by IL-4 on thyroid physiology and disease progression remain largely unknown.
Given the IL-4-mediated induction of DUOX/DUOXA genes expression in thyrocytes (Raad, H. et al. Free Radic. Biol. Med. 56, 216–225 (2013)) and the relative absence of in vivo data concerning the potential impact of IL-4 on thyroid function, we generated a new transgenic mouse model (Thyr-IL-4) expressing this cytokine specifically in thyrocytes. Thyroid function was not strongly modified in Thyr-IL-4 mice with T4 and TSH levels in normal ranges with no visible goiter. However, whole transcriptome sequencing revealed modulation of multiple genes involved in inflammation (Eskalli, Z. et al. Thyroid 26, 1499–1512 (2016)).
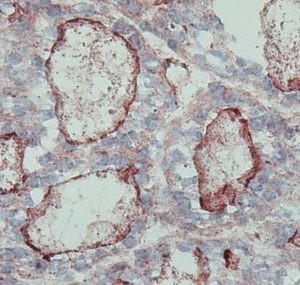
To investigate the role of IL-4 in GD physiopathology, we used experimental models based on F1 hybrids (BALB/c – C57BL/6) mice. To break tolerance, females F1 WT and Thyr-IL-4 mice were immunized against the human TSHr using adenovirus (Ad-hTSHr). The control group received Adenovirus particles expressing the β-galactosidase (Ad-Ctrl). The impact of IL-4 overexpression will be evaluated at multiple levels: induction of hyperthyroidism by total T4 measurements and TSAbs (TSHr stimulating antibodies) and TBAbs (TSHr blocking antibodies) activities ; leukocyte infiltration by immunohistochemical staining.
NOD.H2h4 mice spontaneously developed pathogenic autoimmune thyroiditis that could be accelerated under NaI (0.05%) treatment. Recently, we have generated Thyr-IL-4 mice in the genetic background of NOD.H2h4. Both males and females NOD.H2h4 IL-4 and WT animals received 0.05% NaI in their drinking water during 16 weeks. We will measure the presence of autoantibodies anti-mTG in the animal sera and check if their levels correlate with lymphocytic lesions observed in the thyroid tissues. We will also try to identify the different populations of infiltrated immune cells using classical immunostaining methods and evaluate if the intrathyroidal IL-4 expression has an impact on the pathophysiology.
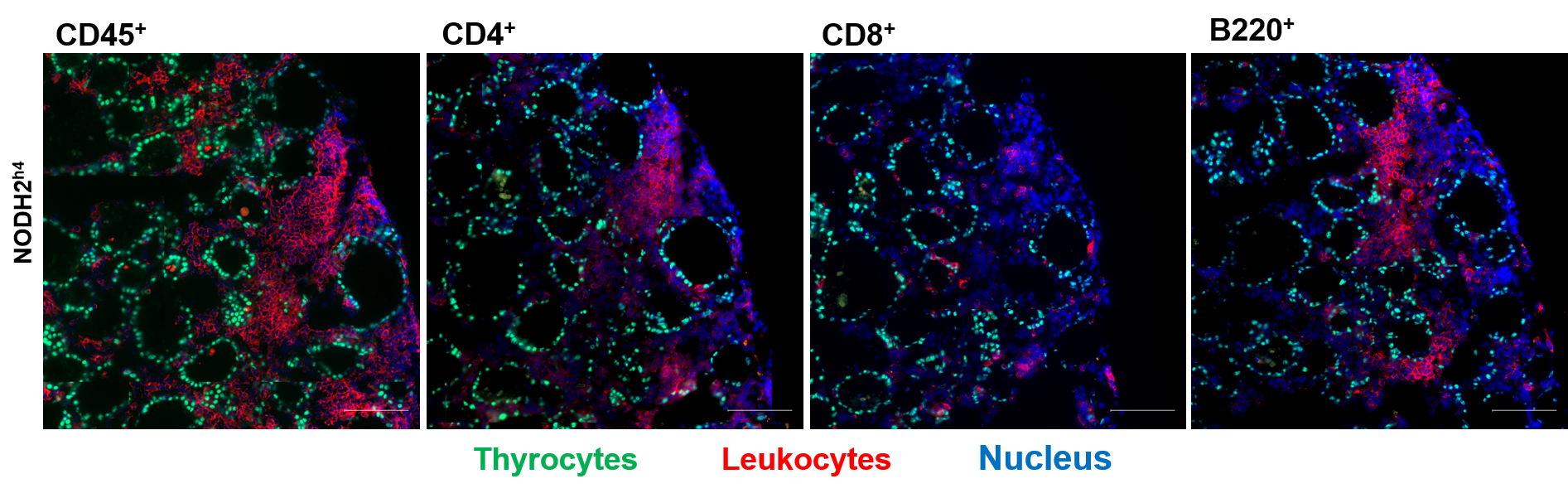
Group members
Principal Investigator
 Xavier De Deken, PhD (xdedeken@ulb.ac.be)
Xavier De Deken, PhD (xdedeken@ulb.ac.be)
phone # +32 (0)2 555 4152
Xavier De Deken obtained a Master’s degree in Biomedical Sciences from ULB (1997). After his PhD in Biomedical Sciences “Cloning and the characterization of the thyroid H2O2 generating system (2002 – ULB), Xavier performed a postdoctoral training at the IRCM, Montreal, Canada (2002 – 2004). Xavier joined back the laboratory in 2004 as a postdoctoral fellow. He obtained a permanent research position in the lab in 2010. His main research interests concern the biochemical characterization of the DUOX oxidases and their role in pathophysiological processes.
![]()
PhD Students :
Sami Djerbib
 Sami Djerbib obtained his veterinary diploma at the National Veterinary School of Algiers (ENSV) in 2015 and completed a Master of specialization at the University of Liège in 2016. He started his PhD in Biomedical Sciences in 2018 at ULB and joined the lab. His main research concerns the study of the implication of hydrogen peroxide (H2O2) in thyroid cancers using a new mouse model overexpressing the H2O2 generator DUOX/DUOXA in the thyroid tissue.
Sami Djerbib obtained his veterinary diploma at the National Veterinary School of Algiers (ENSV) in 2015 and completed a Master of specialization at the University of Liège in 2016. He started his PhD in Biomedical Sciences in 2018 at ULB and joined the lab. His main research concerns the study of the implication of hydrogen peroxide (H2O2) in thyroid cancers using a new mouse model overexpressing the H2O2 generator DUOX/DUOXA in the thyroid tissue.
![]()
Zèna Shourbaji

Zéna Shourbaji obtained her Master’s degree in Biomedical Sciences from ULB (2023). She started her PhD in Biomedical Sciences in 2023 at IRIBHM-ULB. The principal aim of her project is to better decipher the molecular events underlying the development of thyroid follicular cells (TFCs) via an unbiased approach using a pooled-library CRISPR/Cas9 screen on embryonic stem cell (ESC) derived thyroid organoids following the differentiation protocol developed by the laboratory of Pr. S. Costagliola (IRIBHM, ULB).
![]()
Alumnis :
Miot Françoise
Corvilain Bernard
Wang Dantong, obtained his PhD in 2004
Rigutto Sabrina, obtained her PhD in 2009
Hoste Candice, obtained her PhD in 2011
Burniat Agnès, obtained her PhD in 2012
Driessens Natacha, obtained her PhD in 2013
Ghaddhab Chiraz, obtained her PhD in 2014
Eskalli Zineb, obtained her PhD in 2017
Poncelet Louise, obtained her PhD in 2019
Kyrilli Elina, obtained her PhD in 2020
Giusti Nicoletta, obtained her PhD in 2022
Merachi Karima, obtained her PhD in 2023
Publications
Merakchi K, Djerbib S, Soleimani M, Dumont JE, Miot F, De Deken X., “Murine Thyroid IL-4 Expression Worsens Hypothyroidism on Iodine Restriction and Mitigates Graves Disease Development.” Endocrinology. 2022 Sep 1;163(9):bqac107. doi: 10.1210/endocr/bqac107.PMID: 35881515
Kyrilli, A., Gacquer, D., Detours, V., Lefort, A., Libert, F., Twyffels, L., Van Den Eeckhaute, L., Strickaert, A., Maenhaut, C., De Deken, X., Dumont, J.E., Miot, F., Corvilain, B., 2020. Dissecting the Role of Thyrotropin in the DNA Damage Response in Human Thyrocytes after 131I, B Radiation and H2O2. J. Clin. Endocrinol. Metab. 105, 839–853. https://doi.org/10.1210/clinem/dgz185
Giusti, N., Gillotay, P., Trubiroha, A., Opitz, R., Dumont, J.E., Costagliola, S., De Deken, X., 2020. Inhibition of the thyroid hormonogenic H2O2 production by Duox/DuoxA in zebrafish reveals VAS2870 as a new goitrogenic compound. Mol. Cell. Endocrinol. 500, 110635. https://doi.org/10.1016/j.mce.2019.110635
Poncelet L, Dumont JE, Miot F, De Deken X. The Dual Oxidase Duox2 stabilized with DuoxA2 in an enzymatic complex at the surface of the cell produces extracellular H2O2 able to induce DNA damage in an inducible cellular model. Exp Cell Res. 2019 Sep 9:111620.
De Deken X, Miot F.DUOX Defects and Their Roles in Congenital Hypothyroidism.Methods Mol Biol. 2019;1982:667-693. doi: 10.1007/978-1-4939-9424-3_37.
Diebold BA, Wilder SG, De Deken X, Meitzler JL, Doroshow JH, McCoy JW, Zhu Y, Lambeth JD.Guidelines for the Detection of NADPH Oxidases by Immunoblot and RT-qPCR. Methods Mol Biol. 2019;1982:191-229. doi: 10.1007/978-1-4939-9424-3_12.
Dufort G, Larrivée-Vanier S, Eugène D, De Deken X, Seebauer B, Heinimann K, Lévesque S, Gravel S, Szinnai G, Van Vliet G, Deladoëy J. Wide Spectrum of DUOX2 Deficiency: From Life-Threatening Compressive Goiter in Infancy to Lifelong Euthyroidism. Thyroid. 2019 Jul;29(7):1018-1022. doi: 10.1089/thy.2018.0461. Epub 2019 May 31. Erratum in: Thyroid. 2019 Sep;29(9):1347.
Virreira M, Jin L, Djerbib S, De Deken X, Miot F, Massart C, Svoboda M, Van Sande J, Beauwens R, Dumont JE, Boom A. Expression, Localization, and Regulation of the Sodium Bicarbonate Cotransporter NBCe1 in the Thyroid. Thyroid. 2019 Feb;29(2):290-301.
Ghaddhab C, Kyrilli A, Driessens N, Van Den Eeckhaute E, Hancisse O, De Deken X, Dumont JE, Detours V, Miot F, Corvilain B. Factors contributing to the resistance of the thyrocyte to hydrogen peroxide. Mol Cell Endocrinol. 2019 Feb 5;481:62-70. doi: 10.1016/j.mce.2018.11.010. Epub 2018 Nov 23.
Trubiroha A, Gillotay P, Giusti N, Gacquer D, Libert F, Lefort A, Haerlingen B, De Deken X, Opitz R, Costagliola S. A Rapid CRISPR/Cas-based Mutagenesis Assay in Zebrafish for Identification of Genes Involved in Thyroid Morphogenesis and Function. Sci Rep. 2018 Apr 4;8(1):5647.
Dickinson JD, Sweeter JM, Warren KJ, Ahmad IM, De Deken X, Zimmerman MC, Brody SL. Autophagy regulates DUOX1 localization and superoxide production in airway epithelial cells during chronic IL-13 stimulation. Redox Biol. 2018 Apr;14:272-284. doi: 10.1016/j.redox.2017.09.013. Epub 2017 Sep 22. PubMed PMID: 28982074; PubMed Central PMCID: PMC5635347.
Strickaert A, Saiselet M, Dom G, De Deken X, Dumont JE, Feron O, Sonveaux P, Maenhaut C. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene. 2017 May 11;36(19):2637-2642. doi: 10.1038/onc.2016.411. Epub 2016 Oct 31. Review. PubMed PMID: 27797377; PubMed Central PMCID: PMC5442421.
Eskalli Z, Achouri Y, Hahn S, Many MC, Craps J, Refetoff S, Liao XH, Dumont JE, Van Sande J, Corvilain B, Miot F, De Deken X. Overexpression of Interleukin-4 in the Thyroid of Transgenic Mice Upregulates the Expression of Duox1 and the Anion Transporter Pendrin. Thyroid. 2016 Oct;26(10):1499-1512. PubMed PMID: 27599561; PubMed Central PMCID: PMC5067804.
Ameziane-El-Hassani R, Talbot M, de Souza Dos Santos MC, Al Ghuzlan A, Hartl D, Bidart JM, De Deken X, Miot F, Diallo I, de Vathaire F, Schlumberger M, Dupuy C. NADPH oxidase DUOX1 promotes long-term persistence of oxidative stress after an exposure to irradiation. Proc Natl Acad Sci U S A. 2015 Apr 21;112(16):5051-6. doi: 10.1073/pnas.1420707112. Epub 2015 Apr 6. PubMed PMID: 25848056; PubMed Central PMCID: PMC4413347.
5: De Deken X, Corvilain B, Dumont JE, Miot F. Roles of DUOX-mediated hydrogen peroxide in metabolism, host defense, and signaling. Antioxid Redox Signal. 2014 Jun 10;20(17):2776-93. doi: 10.1089/ars.2013.5602. Epub 2013 Oct 25. Review. PubMed PMID: 24161126.
6: Fink K, Martin L, Mukawera E, Chartier S, De Deken X, Brochiero E, Miot F, Grandvaux N. IFNβ/TNFα synergism induces a non-canonical STAT2/IRF9-dependent pathway triggering a novel DUOX2 NADPH oxidase-mediated airway antiviral response. Cell Res. 2013 May;23(5):673-90. doi: 10.1038/cr.2013.47. Epub 2013 Apr 2. Erratum in: Cell Res. 2014 Apr;24(4):509. PubMed PMID: 23545780; PubMed Central PMCID: PMC3641604.
7: Hadad I, Veithen A, Springael JY, Sotiropoulou PA, Mendes Da Costa A, Miot F, Naeije R, De Deken X, Entee KM. Stroma cell-derived factor-1α signaling enhances calcium transients and beating frequency in rat neonatal cardiomyocytes. PLoS One. 2013;8(2):e56007. doi: 10.1371/journal.pone.0056007. Epub 2013 Feb 27. PubMed PMID: 23460790; PubMed Central PMCID: PMC3584107.
Raad H, Eskalli Z, Corvilain B, Miot F, De Deken X. Thyroid hydrogen peroxide production is enhanced by the Th2 cytokines, IL-4 and IL-13, through increased expression of the dual oxidase 2 and its maturation factor DUOXA2. Free Radic Biol Med. 2013 Mar;56:216-25. doi: 10.1016/j.freeradbiomed.2012.09.003. Epub 2012 Sep 23. PubMed PMID: 23010498.
Hoste C, Dumont JE, Miot F, De Deken X. The type of DUOX-dependent ROS production is dictated by defined sequences in DUOXA. Exp Cell Res. 2012 Nov 1;318(18):2353-64. doi: 10.1016/j.yexcr.2012.07.007. Epub 2012 Jul 16. PubMed PMID: 22814254.
Grasberger H, De Deken X, Mayo OB, Raad H, Weiss M, Liao XH, Refetoff S. Mice deficient in dual oxidase maturation factors are severely hypothyroid. Mol Endocrinol. 2012 Mar;26(3):481-92. doi: 10.1210/me.2011-1320. Epub 2012 Feb 2. PubMed PMID: 22301785; PubMed Central PMCID: PMC3286189.
Donkó A, Ruisanchez E, Orient A, Enyedi B, Kapui R, Péterfi Z, de Deken X, Benyó Z, Geiszt M. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic Biol Med. 2010 Dec 15;49(12):2040-8. doi: 10.1016/j.freeradbiomed.2010.09.027. PubMed PMID: 21146788.
Kwon J, Shatynski KE, Chen H, Morand S, de Deken X, Miot F, Leto TL, Williams MS. The nonphagocytic NADPH oxidase Duox1 mediates a positive feedback loop during T cell receptor signaling. Sci Signal. 2010 Aug 3;3(133):ra59. doi: 10.1126/scisignal.2000976. PubMed PMID: 20682913; PubMed Central PMCID: PMC2941205.
Hoste C, Rigutto S, Van Vliet G, Miot F, De Deken X. Compound heterozygosity for a novel hemizygous missense mutation and a partial deletion affecting the catalytic core of the H2O2-generating enzyme DUOX2 associated with transient congenital hypothyroidism. Hum Mutat. 2010 Apr;31(4):E1304-19. doi: 10.1002/humu.21227. PubMed PMID: 20187165.
Dumont JE, De Deken X, Miot F, Corvilain V, Contempré B, Goyens R, Massart C, Van Sande J, Allaoui A, Botteaux A. H2O2, signal, substrate, mutagen and chemorepellent from physiology to biochemistry and disease. Bull Mem Acad R Med Belg. 2010;165(5-6):231-4; discussion 235. PubMed PMID: 21510483.
Song Y, Ruf J, Lothaire P, Dequanter D, Andry G, Willemse E, Dumont JE, Van Sande J, De Deken X. Association of duoxes with thyroid peroxidase and its regulation in thyrocytes. J Clin Endocrinol Metab. 2010 Jan;95(1):375-82. doi: 10.1210/jc.2009-1727. Epub 2009 Dec 1. PubMed PMID: 19952225.
Allaoui A, Botteaux A, Dumont JE, Hoste C, De Deken X. Dual oxidases and hydrogen peroxide in a complex dialogue between host mucosae and bacteria. Trends Mol Med. 2009 Dec;15(12):571-9. doi: 10.1016/j.molmed.2009.10.003. Epub 2009 Nov 11. PubMed PMID: 19913458.
Driessens N, Versteyhe S, Ghaddhab C, Burniat A, De Deken X, Van Sande J, Dumont JE, Miot F, Corvilain B. Hydrogen peroxide induces DNA single- and double-strand breaks in thyroid cells and is therefore a potential mutagen for this organ. Endocr Relat Cancer. 2009 Sep;16(3):845-56. doi: 10.1677/ERC-09-0020. Epub 2009 Jun 9. PubMed PMID: 19509065.
Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, De Deken X. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by camp-dependent protein kinase and protein kinase C-dependent phosphorylation. J Biol Chem. 2009 Mar 13;284(11):6725-34. doi: 10.1074/jbc.M806893200. Epub 2009 Jan 14. PubMed PMID: 19144650; PubMed Central PMCID: PMC2652333.
Znaidi S, Weber S, Al-Abdin OZ, Bomme P, Saidane S, Drouin S, Lemieux S, De Deken X, Robert F, Raymond M. Genomewide location analysis of Candida albicans Upc2p, a regulator of sterol metabolism and azole drug resistance. Eukaryot Cell. 2008 May;7(5):836-47. doi: 10.1128/EC.00070-08. Epub 2008 Apr 4. PubMed PMID: 18390649; PubMed Central PMCID: PMC2394978.
Znaidi S, De Deken X, Weber S, Rigby T, Nantel A, Raymond M. The zinc cluster transcription factor Tac1p regulates PDR16 expression in Candida albicans. Mol Microbiol. 2007 Oct;66(2):440-52. PubMed PMID: 17897373.
Song Y, Driessens N, Costa M, De Deken X, Detours V, Corvilain B, Maenhaut C, Miot F, Van Sande J, Many MC, Dumont JE. Roles of hydrogen peroxide in thyroid physiology and disease. J Clin Endocrinol Metab. 2007 Oct;92(10):3764-73. Epub 2007 Jul 31. Review. PubMed PMID: 17666482.
Rigutto S, Hoste C, Dumont JE, Corvilain B, Miot F, De Deken X. Duox1 is the main source of hydrogen peroxide in the rat thyroid cell line PCCl3. Exp Cell Res. 2007 Nov 1;313(18):3892-901. Epub 2007 Jun 29. PubMed PMID: 17643428.
Grasberger H, De Deken X, Miot F, Pohlenz J, Refetoff S. Missense mutations of dual oxidase 2 (DUOX2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with DUOX2 maturation factor. Mol Endocrinol. 2007 Jun;21(6):1408-21. Epub 2007 Mar 20. PubMed PMID: 17374849.
Milenkovic M, De Deken X, Jin L, De Felice M, Di Lauro R, Dumont JE, Corvilain B, Miot F. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol. 2007 Mar;192(3):615-26. PubMed PMID: 17332529.
Saidane S, Weber S, De Deken X, St-Germain G, Raymond M. PDR16-mediated azole resistance in Candida albicans. Mol Microbiol. 2006 Jun;60(6):1546-62. PubMed PMID: 16796687.
MacPherson S, Akache B, Weber S, De Deken X, Raymond M, Turcotte B. Candida albicans zinc cluster protein Upc2p confers resistance to antifungal drugs and is an activator of ergosterol biosynthetic genes. Antimicrob Agents Chemother. 2005 May;49(5):1745-52. PubMed PMID: 15855491; PubMed Central PMCID: PMC1087678.
Wang D, De Deken X, Milenkovic M, Song Y, Pirson I, Dumont JE, Miot F. Identification of a novel partner of duox: EFP1, a thioredoxin-related protein. J Biol Chem. 2005 Jan 28;280(4):3096-103. Epub 2004 Nov 22. PubMed PMID: 15561711.
De Deken X, Raymond M. Constitutive activation of the PDR16 promoter in a Candida albicans azole-resistant clinical isolate overexpressing CDR1 and CDR2. Antimicrob Agents Chemother. 2004 Jul;48(7):2700-3. PubMed PMID: 15215129; PubMed Central PMCID: PMC434214.
De Deken X, Wang D, Dumont JE, Miot F. Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp Cell Res. 2002 Feb 15;273(2):187-96. PubMed PMID: 11822874.
De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem. 2000 Jul 28;275(30):23227-33. PubMed PMID: 10806195.
De Deken X, Vilain C, Van Sande J, Dumont JE, Miot F. Decrease of telomere length in thyroid adenomas without telomerase activity. J Clin Endocrinol Metab. 1998 Dec;83(12):4368-72. PubMed PMID: 9851779.