Our research

The main research theme of our laboratory is the study of the major regulators and mechanisms regulating cardioprotection and the response to cardiac ischemia. We investigate actively the role of the mouse cardiac adipose tissue in cardioprotection and as a source of cardiac stem cells.
 The starting point of our projects is the analysis of the cardiac phenotype of mice deficient for extracellular nucleotide receptors and cardioprotective adipokines. We acquired previously an expertise in the field of extracellular nucleotides and pharmacology of GPCRs by the cloning and the study of human P2Y nucleotide receptors.
The starting point of our projects is the analysis of the cardiac phenotype of mice deficient for extracellular nucleotide receptors and cardioprotective adipokines. We acquired previously an expertise in the field of extracellular nucleotides and pharmacology of GPCRs by the cloning and the study of human P2Y nucleotide receptors.
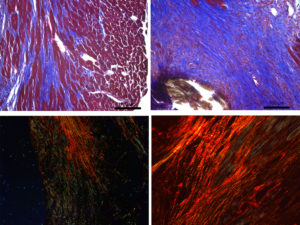
The cardiac adipose tissue displays anatomical and functional contiguity to the myocardium and coronary arteries. The emerging pathophysiological aspects of cardiac fat have multiple innovative clinical applications. Cardiac fat has cardioprotective properties such as adiponectin secretion but can also locally affect the heart through secretion of proinflammatory cytokines.
Rec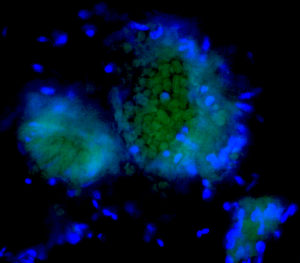 ently fat-associated lymphoid clusters (FALCs) have been identified in adipose tissues as areas containing innate-like B and T cell populations. The formation of these clusters can be induced by inflammation and ischemia, and associated to rapid immune responses. The exact composition, distribution, function of FALCs remain unknown. Interestingly we observed that P2Y nucleotide receptors are involved in protection against myocardial infarction, in formation of heart, cardiac fat and FALCs, and finally in secretion of cardioprotective adipokines.
ently fat-associated lymphoid clusters (FALCs) have been identified in adipose tissues as areas containing innate-like B and T cell populations. The formation of these clusters can be induced by inflammation and ischemia, and associated to rapid immune responses. The exact composition, distribution, function of FALCs remain unknown. Interestingly we observed that P2Y nucleotide receptors are involved in protection against myocardial infarction, in formation of heart, cardiac fat and FALCs, and finally in secretion of cardioprotective adipokines.
Our projects combine in vitro studies on isolated cardiac cells and stem cells, mouse in vivo ischemia models and study of polymorphisms of human genes involved in cardioprotection. The study of receptors regulating cardiac fat mass and expression of cardioprotective proteins is very promising. The cardiac adipose tissue is a known source of stem cells allowing the restoring of cardiac function after myocardial infarction and having thus therapeutical applications.
The main projects are:
– Study of the mechanisms involved in the formation and cardioprotective role of cardiac adipose tissue and its fat-associated lymphoid clusters (FALCs).
– Post-ischemic revascularization of the heart after injection of nucleotide-treated adipose-derived stem cells in the ischemic heart.
– Identification and characterization of target genes of P2Y and adipokines in cardiac fat in basal and ischemic mice using left artery descending artery (LAD) ligation model.
– Study of polymorphisms in P2Y human genes in a large cohort of patients displaying cardiovascular disorders.
Group members
Didier Communi, PI, Research Associate FNRS (communid@ulb.ac.be)
phone # +32 (0) 2 555 4159
Michael Horckmans, Post-doc
Cardiac ischemia and inflammation in vivo models, cardioprotective role of adipokines
Irène Negri, PhD student
Role of nucleotide receptors in cardiac fat formation and post-ischemic expansion of fat-associated lymphoid clusters
Esteban Diaz Villamil, Master Student
Lucas De Roeck, Technician
Publications
Main Publications
« Loss-of-function N178T variant of the human P2Y4 receptor is associated with decreased severity of coronary artery disease and improved glucose homeostasis. » Michael Horckmans, Esteban Diaz Villamil, Céline Verdier, Henrik Laurell, H., Jean-Bernard Ruidavets, Lucas De Roeck, Guillaume Combes, Laurent O. Martinez and Didier Communi. Frontiers in Pharmacology (2022) https://doi.org/10.3389/fphar.2022.1049696
“Central role of PD-L1 in cardioprotection resulting from P2Y4 nucleotide receptor loss.” Michael Horckmans, Esteban Diaz Villamil, Mariaelvy Bianchini, Lucas De Roeck and Didier Communi. Frontiers in Immunology (2022). https://doi.org/10.3389/fimmu.2022.1006934
« UTP regulates the cardioprotective action of transplanted stem cells derived from mouse cardiac adipose tissue. » Esteban Diaz Villamil, Lucas De Roeck, Marion Vanorlé and Didier Communi. Frontiers in Pharmacology (2022) https://doi.org/10.3389/fphar.2022.906173
Lemaire, A., Vanorlé, M., Horckmans, M., di Pietrantonio, L., Clouet, S., Robaye, B., Boeynaems, J.M., Communi, D. Mouse P2Y4 nucleotide receptor is a negative regulator of cardiac adipose-derived stem celll differentiation and cardiac fat formation. Stem Cells Dev., 2016, 26(5) : 363-373.
Clouet, S., Di Pietrantonio, L., Daskalopoulos, E.P., Esfahani, H., Horckmans, M., Vanorlé, M., Lemaire, A., Balligand, J.-L., Beauloye, C., Boeynaems, J.-M., Communi, D. Loss of mouse P2Y6 nucleotide receptor is associated with physiological macrocardia and amplified pathological cardiac hypertrophy. Journal of Biological Chemistry, 2016, 291(30), 15841-15852.
Horckmans, M., Esfahani, H., Beauloye, C., Clouet, S., di Pietrantonio, L., Robaye, B., Balligand, J.L., Boeynaems, J.M., Dessy, C., Communi, D. Loss of P2Y4 nucleotide receptor protect against myocardial infarction through endothelin-1 downregulation. Journal of Immunology, 2015, 194(4), 1874-1881.
Horckmans, M., Léon-Gomez, E., Robaye, B., Balligand, J.L., Boeynaems, J.M., Dessy, C., Communi, D. Gene deletion of P2Y4 receptor lowers exercise capacity and reduces myocardial hypertrophy with swimming exercise. Am. J. Physiol. Heart Circ. Physiol., 2012, 303(7), H835-H843.
Horckmans M, Robaye B, Léon-Gόmez E, Lantz N, Unger P, Dol-Gleizes F, Cammarata D, Schaeffer P, Savi P, Gachet C, Balligand J-L, Dessy C, Boeynaems J-M, Communi D. P2Y4 nucleotide receptor: a novel actor in post-natal cardiac development and function, Angiogenesis, 2012, 15(3), 349360, 10.1007/s10456-012-9265-1.
Bles, N., Di Pietrantonio, L., Boeynaems, J.-M., Communi, D. ATP confers tumorigenic properties to dendritic cells by inducing amphiregulin secretion. Blood, 2010, 116(17), 3219-3226
Vanderstocken, G., Bondue, B., Horckmans, M., Di Pietrantonio, L., Robaye, B., Boeynaems, J.-M., Communi, D. P2Y2 receptor regulates VCAM-1 membrane and soluble forms and eosinophil accumulation during lung inflammation. Journal of Immunology, 2010, 185(6), 3702-3707
Bles, N., Horckmans, M., Lefort, A., Libert, F., Macours, P., El Housni, H., Marteau, F., Boeynaems, J.M., Communi, D. Gene expression profiling defines ATP as a key regulator of human dendritic cell functions. Journal of Immunology, 2007, 179, 3550-3558
Marcet, B., Libert, F., Boeynaems, J.M., Communi, D. Extracellular nucleotides induce COX-2 upregulation and prostaglandin E2 production in human A549 alveolar type II epithelial cells. European Journal of Pharmacology, 2007, 566, 167-171
Marcet, B., Horckmans, M., Libert, F., Hassid, S., Boeynaems, J.M., Communi, D. Extracellular nucleotides regulate CCL20 release from human primary airway epithelial cells, monocytes and monocyte-derived dendritic cells. Journal of Cellular Physiology, 2007, 211, 716-727
Marteau, F., Suarez Gonzalez, N., Communi, David, Goldman, M., Boeynaems, J.M., Communi, Didier. Thrombospondin-1 and indoleamine 2,3-dioxygenase are major targets of extracellular ATP in human dendritic cells. Blood, 2005, 106, 3860-3866
Autiero, M.*, Waltenberger, J.*, Communi, Didier* (*: first co-authors), Kranz, A., Moons, L., Lambrechts, D., Kroll J, Plaisance S, De Mol M, Bono F, Kliche S, Fellbrich G, Ballmer-Hofer K, Maglione D, Mayr-Beyrle U et al. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nature Medicine, 2003, 9, 936-943
Duhant, X., Schandené, L., Bruyns, C., Suarez Gonzalez, N., Goldman, M., Boeynaems, J.M., Communi, D. Extracellular adenine nucleotides inhibit the activation of human CD4+ T lymphocytes. Journal of Immunology, 2002, 169, 15-21
Communi, D., Suarez Gonzalez, N., Detheux, M., Brézillon, S., Lannoy, V., Parmentier, M., Boeynaems, J.M. Identification of a novel human ADP receptor coupled to G(i). Journal of Biological Chemistry, 2001, 276, 41479-41485.
Communi, D., Suarez-Huerta, N., Dussossoy, D., Savi, P., Boeynaems, J.M. Cotranscription and intergenic splicing of human P2Y11 and SSF1 genes. Journal of Biological Chemistry, 2001, 276, 16561-16566
Communi, D., Boeynaems, J.M. Involvement of distinct receptors in the actions of extracellular uridine nucleotides. Trends in Pharmacological Sciences, 1997, 18, 83-86
Communi, D., Govaerts, C., Parmentier, M., Boeynaems, J.M. Cloning of a human purinergic P2Y receptor coupled to phospholipase C and adenylyl cyclase. Journal of Biological Chemistry, 1997, 272, 31969-31973.
Communi, D., Robaye, B., Boeynaems, J.M. Pharmacological characterization of the human P2Y11 receptor. British Journal of Pharmacology, 1999, 128, 1199-1206
Communi, D., Paindavoine, P., Place, G.A., Parmentier, M., Boeynaems, J.M. Expression of P2Y receptors in cell lines derived from the human lung. British Journal of Pharmacology, 1999, 127, 562-568
Communi, D., Pirotton, S., Parmentier, M., Boeynaems, J.M. Cloning and functional expression of a human uridine nucleotide receptor. Journal of Biological Chemistry, 1995, 270, 30849-30852.
Communi, D., Raspé, E., Pirotton, S., Boeynaems, J.M.
Coexpression of P2Y and P2U receptors on aortic endothelial cells: comparison of cell localization and signaling pathways. Circulation Research, 1995, 76, 191-198