Our research
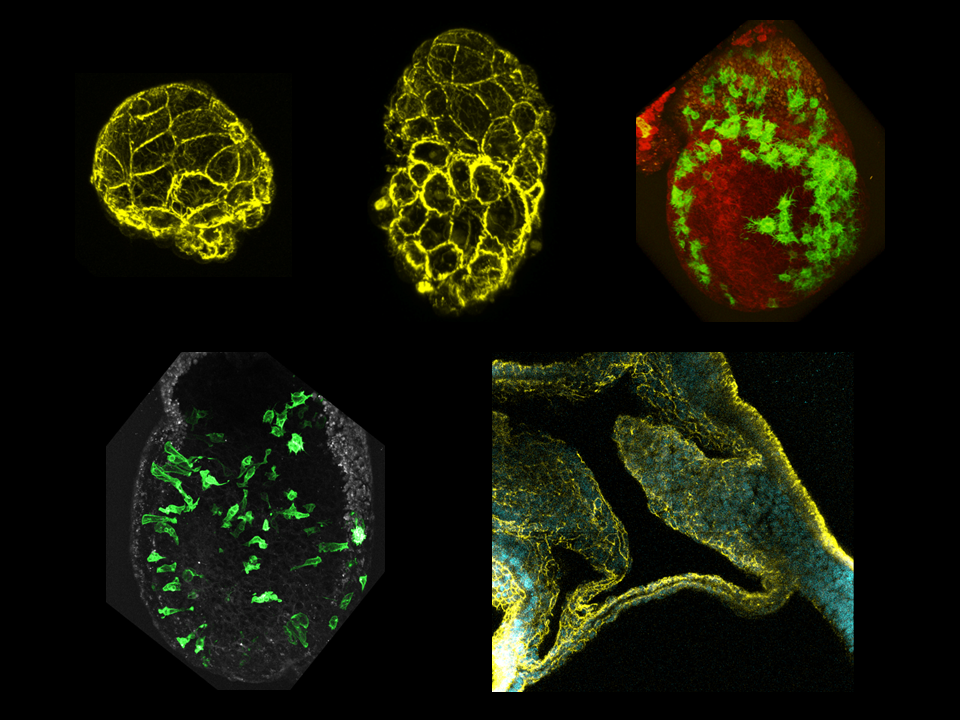
The laboratory is devoted to the study of cell and cytoskeleton dynamics during early mammalian embryo development. We have two main themes of research: the mechanisms of gastrulation Epithelial-Mesenchymal Transition at the Primitive Streak after establishment of the anterior-posterior axis, and the subsequent morphogenesis of mesoderm-derived structures, in particular the extra-embryonic support membranes.
Gastrulation
We developed tools to observe epithelial-mesenchymal migration (EMT) at the Primitive Streak prior to mesoderm formation and identified an asymmetry in mitosis frequency and position. We then used mouse genetics, pharmacological screens, computational simulation and fixed and live imaging of embryos and stem cells-based embryo models to demonstrate a role for mitosis in cell delamination.
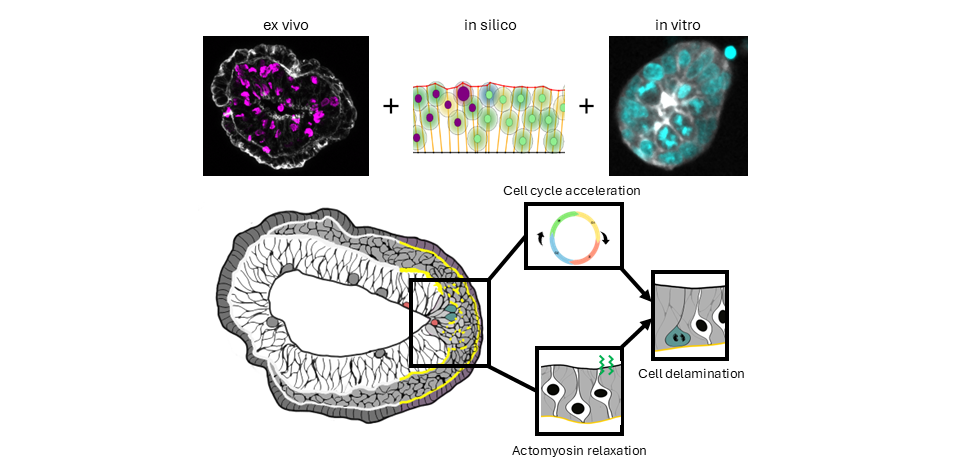
The lab has established techniques for culture and live imaging of post-implantation mouse embryos, as well as mesoderm explants. Mechanistically, we focus on the role of cytoskeleton and mechanical forces. We identified differences in the behavior and cytoskeleton organization of mesoderm cells that participate to either embryonic tissues or extra-embryonic envelopes such as the amnion, umbilical cord, or placenta. We have identified a role for keratin intermediate filaments in the morphogenesis of those membranes.
We use an array of approaches including fixed and live imaging of embryos and tissue explants, omics on cell populations and micro-dissected structures, and exploration of methods recapitulating aspects of the morphogenesis of extra-embryonic envelopes such as stem cells-derived embryo models.
Ongoing projects aim to
- Define the interplay between cells’ cytoskeleton/adhesion repertoire and their mechanical properties
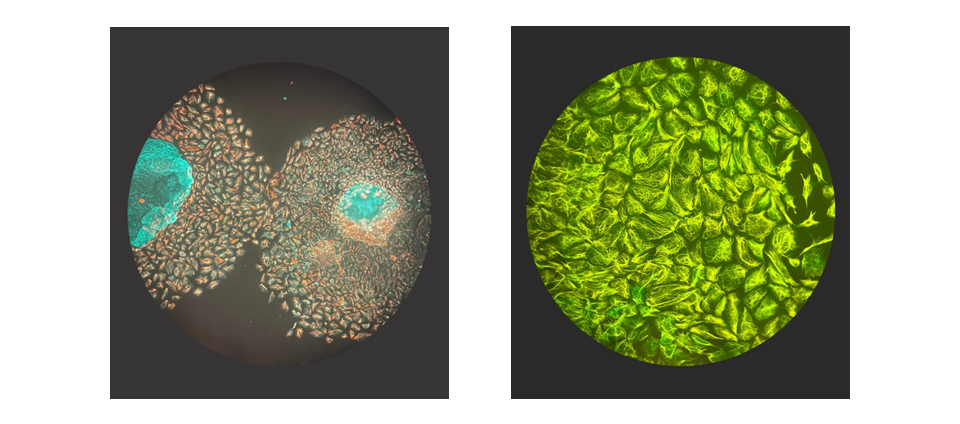
- Explore the anatomical and functional development of the amnion, yolk sac, umbilical cord, and placenta
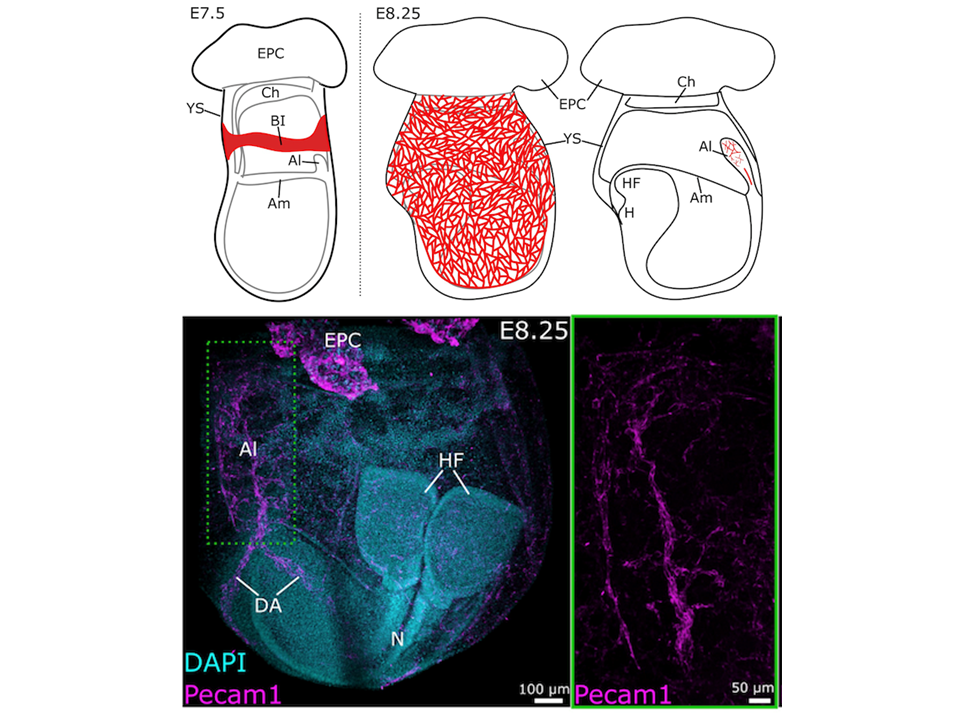
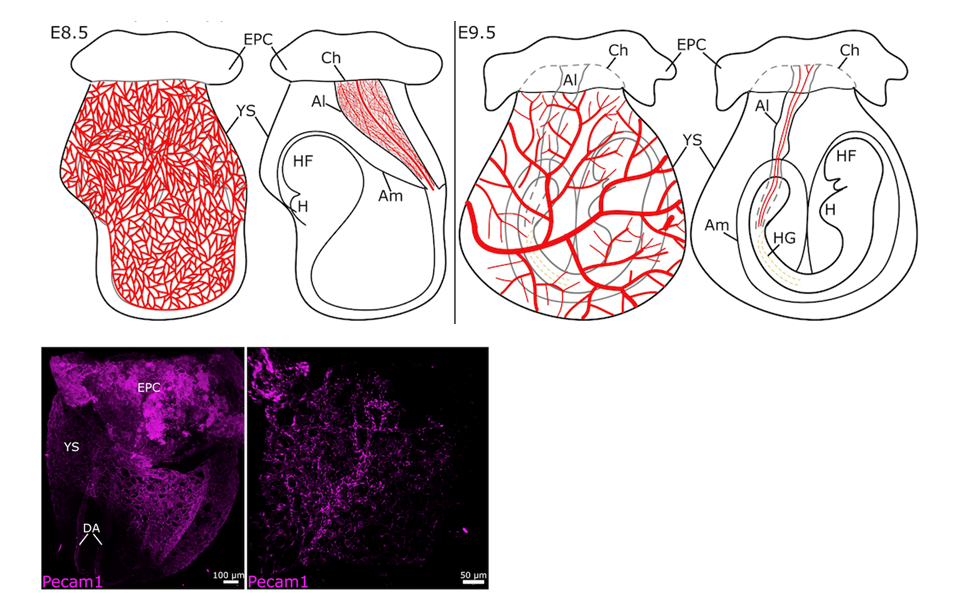
- Characterize the formation of the vitelline and umbilical vasculatures
Funding



Group members
 Isabelle Migeotte – Principal Investigator Research Associate F.N.R.S (isabelle.migeotte@ulb.be)
Isabelle Migeotte – Principal Investigator Research Associate F.N.R.S (isabelle.migeotte@ulb.be)
phone # +32 (0)2 555 4529
I received a MD PhD from Université Libre de Bruxelles, and certified in Internal Medicine and Medical Genetics. I trained in Developmental Biology with Kathryn Anderson in Sloan-Kettering Institute in NYC. I became a FNRS Research Associate and Principal Investigator at IRIBHM in 2012. My laboratory focuses on the cellular mechanisms regulating morphogenesis of the mouse embryo and its extra-embryonic membranes. In parallel, I teach genetics to bachelor students in medicine and biomedical sciences and am a consultant at the ULB Centre of Human Genetics, with clinical (medical genetics consults) and research (diagnostic techniques/ gene discovery in clinical cohorts) interests.
![]()

Eliana Nehme, PhD Student
I graduated from Notre Dame University-Lebanon (NDU) with a Bachelor of Science degree majoring in Medical Laboratory Technology. Afterwards, I was granted a scholarship that enabled me to complete my master’s degree in Biomedical Sciences: Biochemistry and Molecular Genetics at the American University of Beirut-Lebanon (AUB). My work focused on the epigenetic suppression of a group of evolutionary conserved transcription factors, the TBX2 subfamily, in human non-small cell lung cancer. Passionate about research, I joined Isabelle Migeotte’s lab in November 2022 thanks to the International PhD program of IRIBHM, and obtained a FRIA fellowship in 2023. My work focuses on the molecular determinants for the mechanical properties of the mouse embryo extra-embryonic membranes.
Passionate about research, I joined Isabelle Migeotte’s lab in November 2022. My work focuses on the molecular determinants for the mechanical properties of the mouse embryo extra-embryonic membranes.
![]()

Kristof Van Schoor, PhD Student
I obtained a professional bachelor’s degree in medical laboratory technology from the University of Applied Sciences and Art in Ghent in 2018. For my bachelor’s thesis, I studied the effects of decalcification on immunohistochemical staining in bone marrow diagnostics. In 2022, I received a master’s degree in biomedical sciences from the KU Leuven. I wrote my master thesis on canonical BMP signalling in the development of the intestinal lymphatic system with Prof. An Zwijsen at the Center for Molecular and Vascular Biology (KU Leuven). In November 2022, I joined the IRIBHM to study the development of the extraembryonic vasculature and the attachment of the umbilical cord to the placenta
![]()

Sorya Fagnoul, PhD Student
After completing my medical training (ULB) in 2016, I specialized in nephrology in 2022. I joined the Migeotte Lab in October 2022 with a grant from the Fonds Erasme. My project will endeavour to improve diagnosis of kidney diseases of genetical eatiology. In addition to monogenic diseases, I will explore more complex modes of inheritance, such as oligenic diseases, thanks to a novel method created by our colleagues at IB² (Interuniversity Institute of Bioinformatics in Brussels). In kidney transplantation, I will evaluate the value of a genetic diagnosis to reduce renal risk for both donor and recipient, but also maximise their matching to avoid organ rejection. Finally, I aim to establish a platform of renal organoids to test the variants of interest we will identify.
![]()

Emmanuel Bruet , Post-doctoral fellow
I obtained my bachelor’s degree in pharmacology from the Université d’Auvergne in Clermont-Ferrand, France in 2016. In 2019, I received a master’s degree in cellular signalization and neuroscience from the Université Paris-Saclay, France. During my master’s degree, I joined Doctor Sophie Creuzet’s Development and Evolution of the Neural Crest lab at the Paris-Saclay Institute of Neuroscience where I studied the emerging role of the cephalic neural crest-derived meninges and pericytes in cognitive impairment at birth. I pursued my research in the same laboratory with a PhD from 2019 to 2023 after receiving an IDEX grant from the French State. In December 2023, I joined Isabelle Migeotte’s lab to characterize the extra-embryonic vascular circulation through imaging and scRNAseq.
![]()
Alumni:
Wallis Nahaboo, PhD
Evangéline Despin-Guitard, PhD
Latifa Hammou
Bechara Saykali, PhD
Marie-Lucie Racu, MD
Diana Suarez Boomgaard, PhD
Navrita Mathiah, PhD
Agathe Denys
Ayoub Radi, Lab technician
Publications
Despin-Guitard E, Rosa VS, Plunder S, Mathiah N, Van Schoor K, Nehme E, Merino-Aceituno S, Egea J, Shahbazi MN, Theveneau E, Migeotte I. Non-apical mitoses contribute to cell delamination during mouse gastrulation. Nat Commun15, 7364 (2024). https://doi: 10.1038/s41467-024-51638-6
Van Schoor Kristof , Bruet Emmanuel , Jones Elizabeth Anne Vincent , Migeotte Isabelle. Origin and flow-mediated remodeling of the murine and human extraembryonic circulation systems, Frontiers in Physiology, Volume 15 – 2024 |https://doi.org/10.3389/fphys.2024.1395006
Evangéline Despin-Guitard, Ronan Quenec’Hdu, Wallis Nahaboo, Nicole Schwarz, Rudolf E.Leube, Claire Chazaud and Isabelle Migeotte. Frontiers in Cell and Developmental Biology (2022) “Regionally specific levels and patterns of keratin 8 expression in the mouse embryo visceral endoderm emerge upon anterior-posterior axis determination” Frontiers in Cell and Developmental Biology (2022) https://doi.org/10.3389/fcell.2022.1037041
Nahaboo W., Eski S.E., Vermeersch M., Saykali B., Monteyne D., Magin T., Schwarz N., Zwijsen A, Perez-Morga D., Singh S. P. Migeotte I. (2021) . “Keratin dynamics govern the establishment of the maternal-fetal interface”.
The EMBO Journal (2022). e108747. https://doi.org/10.15252/embj.2021108747
Nahaboo W, Saykali B, Mathiah N, Migeotte I. (2021) Visualizing Mouse Embryo Gastrulation Epithelial-Mesenchymal Transition Through Single Cell Labeling Followed by Ex Vivo Whole Embryo Live Imaging. Methods Mol Biol. 2021;2179:135-144. https://doi: 10.1007/978-1-0716-0779-4_12.PMID: 32939718
N. Mathiah, E. Despin-Guitard,https://doi.org/10.3389/fphys.2024.1395006 M. Stower, W. Nahaboo, E. S. Eski, S. P. Singh, S. Srinivas, and I. Migeotte, “Asymmetry in the frequency and position of mitosis in the mouse embryo epiblast at gastrulation”, EMBO Rep, vol. 9, no. n/a, p. 317, Oct. 2020. https://doi: 10.15252/embr.202050944
Q. Zhang et al. “Inborn errors of type I IFN immunity in patients with life-threatening COVID-19,” Science Sep. 2020, p. eabd4570. (Migeotte. I, IRIBHM) https://doi: 10.1126/science.abd4570
Kyprianou, C., Christodoulou, N., Hamilton, R. S., Nahaboo, W., Suárez-Boomgaard, D., Amadei, G., Migeotte, I., & Zernicka-Goetz, M. Basement membrane remodelling regulates mouse embryogenesis. (2020) Nature (London), 582(7811), 253-258. https://doi:10.1038/s41586-020-2264-2
Delavallée, L., Mathiah, N., Cabon, L., Mazeraud, A., Brunelle-Navas, M.-N., Lerner,L. L., Tannoury, M., Prola, A., Moreno-Loshuertos, R., Baritaud, M., Vela, L., Garbin, K., Garnier, D., Lemaire, C., Langa-Vives,F., Cohen-Salmon, M., Fernández-Silva, P., Chretien, F., Migeotte, I., & Susin, S. A. Mitochondrial AIF loss causes metabolic reprogramming, caspase-independent cell death blockade, embryonic lethality, and perinatal hydrocephalus (2020). M. Molecular metabolism, 40, 101027. https://doi:10.1016/ j.molmet.2020.101027
Saykali, B., Nahaboo, W., Mathiah, N., Racu, M.-L., Defrance, M., & Migeotte, I. (2019). Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. eLife, 8, e42434. https://doi:10.7554/eLife.42434.001
Nahaboo, Wallis, and Migeotte, Isabelle (2018) Cleavage and Gastrulation in the Mouse Embryo. In: eLS. John Wiley & Sons Ltd, Chichester. http://www.els.net doi:10.1002/9780470015902.a0001068.pub3
Browet, A. A., De Vleeschouwer, C., Jacques, L. L., Mathiah, N., Saykali, B., & Migeotte, I. (2016). Cell segmentation with random ferns and graph-cuts. Proceedings – International Conference on Image Processing. 7533140, 4145-4149. https://doi:10.1109/ICIP.2016.7533140.
Mazari, E., Zhao, X., Migeotte, I., Collignon, J., Gosse, C., & Perea-Gomez, A. (2014). A microdevice to locally electroporate embryos with high efficiency and reduced cell damage. Development, , dev.106633. http://doi.org/10.1242/dev.106633
Zhao, X., Mazari, E., Suárez-Boomgaard, D. D., Migeotte, I., Perea-Gomez, A., & Gosse, C. (2014). Finite element model simulations to assist the design of microdevices dedicated to the localized electroporation of mouse embryos. E C S Transactions, 64(16), 7 14. https://doi:10.1149/06416.0007ecst
Simonis, N., Migeotte, I., Lambert, N., Perazzolo, C., de Silva, D. C., Dimitrov, B., Heinrichs, C., Janssens, S., Kerr, B., Mortier, G., Van Vliet, G., Lepage, P., Casimir, G., Abramowicz, M., Smits, G., & Vilain, C. (2013, June). FGFR1 mutations cause Hartsfield syndrome, the unique association of holoprosencephaly and ectrodactyly. Journal of medical genetics, 50(9), 585-592. https://doi:10.1136/jmedgenet-2013-101603
Bloomekatz, J., Grego-Bessa, J., Migeotte, I., & Anderson, K. V. (2012). Pten regulates collective cell migration during specification of the anterior-posterior axis of the mouse embryo. Developmental Biology, 364(2), 192–201. http://doi.org/10.1016/j.ydbio.2012.02.005
Migeotte, I., Grego-Bessa, J., & Anderson, K. V. (2011). Rac1 mediates morphogenetic responses to intercellular signals in the gastrulating mouse embryo. Development (Cambridge, England), 138(14), 3011–3020. http://doi.org/10.1242/dev.059766
Lee, J. D., Migeotte, I., & Anderson, K. V. (2010). Left-right patterning in the mouse requires Epb4.1l5-dependent morphogenesis of the node and midline. Developmental Biology, 346(2), 237–246. http://doi.org/10.1016/j.ydbio.2010.07.029
Migeotte, I., Omelchenko, T., Hall, A., & Anderson, K. V. (2010). Rac1-dependent collective cell migration is required for specification of the anterior-posterior body axis of the mouse. PLoS Biology, 8(8), 37–38. http://doi.org/10.1371/journal.pbio.1000442